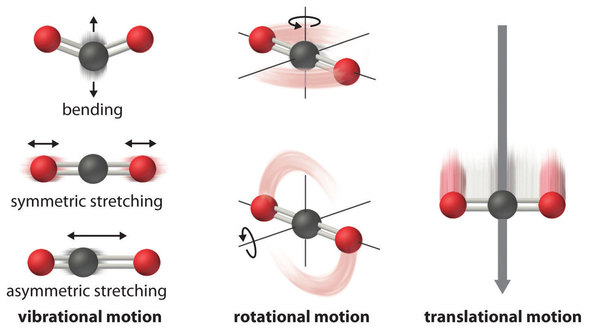
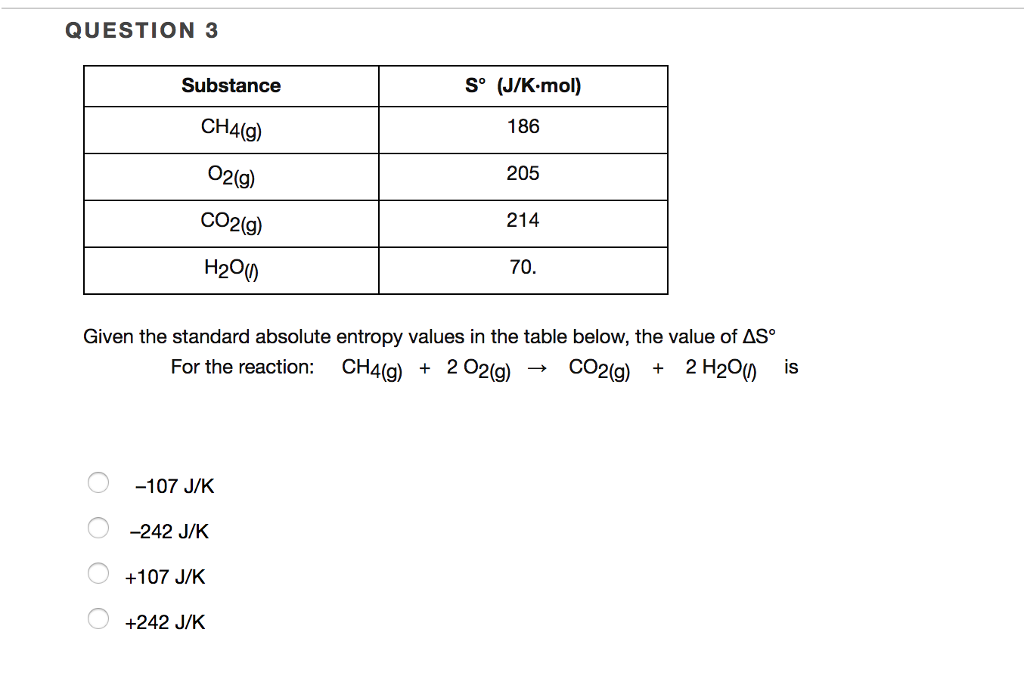
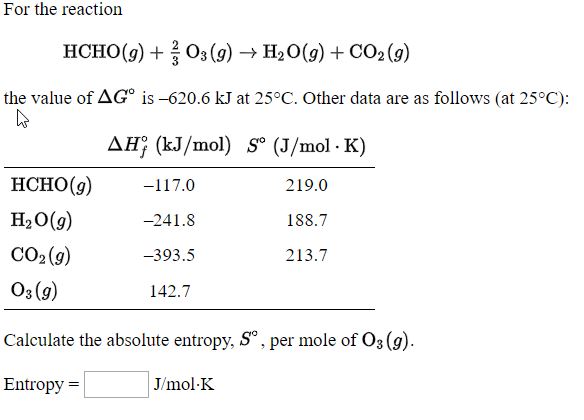
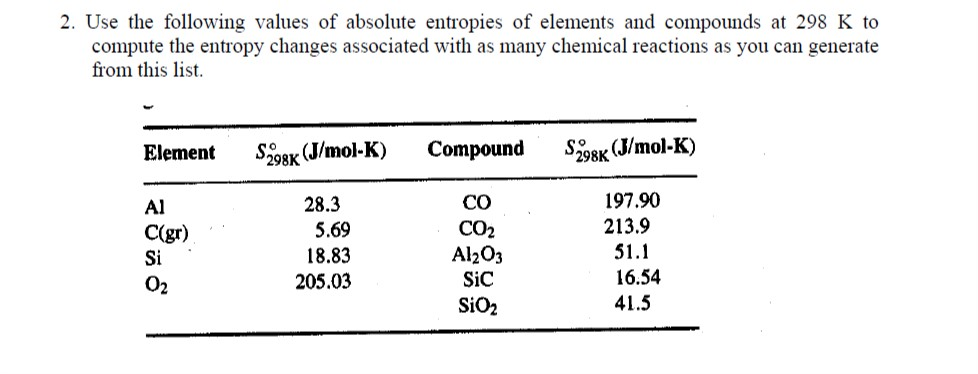
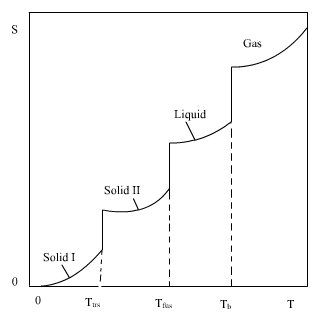
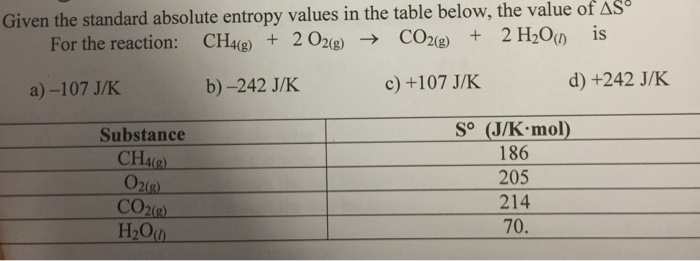Why is negative the absolute entropy, enthalpy and internal energy in solids on the sublimation curve (steam properties)? | ResearchGate

Determination of Absolute Entropy Values for Solid, Liquid & Gases with third law of thermodynamics. - YouTube

SOLVED: * Required 1. Calculate IS? at 25*C for the reduction of Pbo(s), 2Pbo(s) + C(s) > 2Pb(s) + CO2(g) given these absolute entropies S' (J/K-mol) Entropy values Pbo(s)= 69.45 C(s) =

Standard Absolute Entropy, , Values from Volume or Density. 1. Inorganic Materials | Inorganic Chemistry

SOLVED: Without doing any calculations or looking up absolute entropy values, determine the sign of ASsys and ASsurr for each of the following chemical reactions below. In addition, predict under what temperatures (

Standard Absolute Entropy, , Values from Volume or Density. 1. Inorganic Materials | Inorganic Chemistry

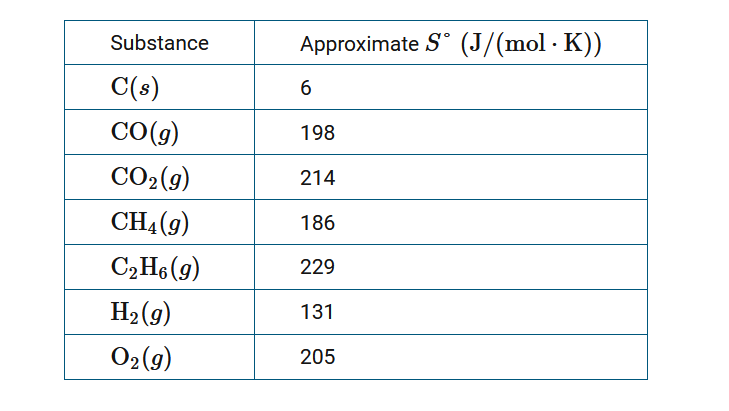
OneClass: Calculate the standard entropy change for the reaction P_4(g) + 5O_2(g) rightarrow P_4 O_10...
![The Determination of Absolute Values of Entropies of Hydration [ΔSabs0(H+)h] and Aquation [ΔSabs0(H+)aq] and The Thermodynamics of Proton in Solutions The Determination of Absolute Values of Entropies of Hydration [ΔSabs0(H+)h] and Aquation [ΔSabs0(H+)aq] and The Thermodynamics of Proton in Solutions](https://www.degruyter.com/document/doi/10.1515/zpch-2016-0867/asset/graphic/j_zpch-2016-0867_fig_001.jpg)