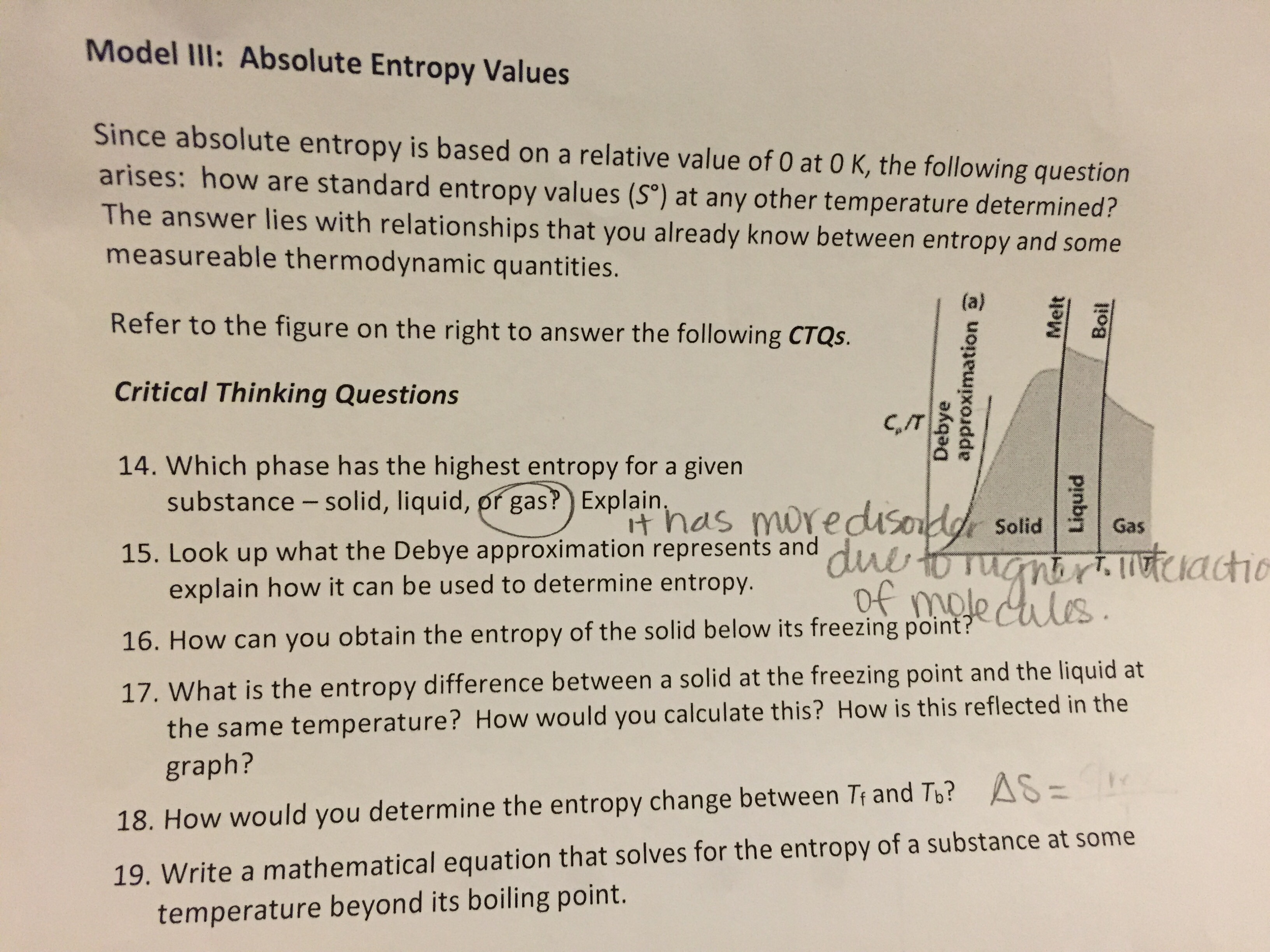
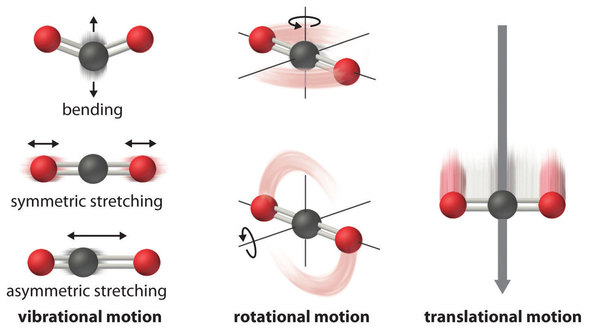
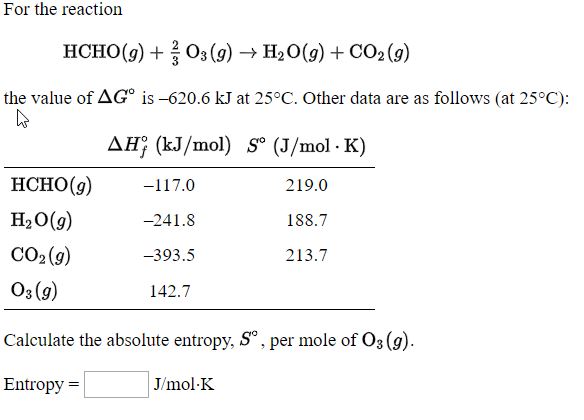
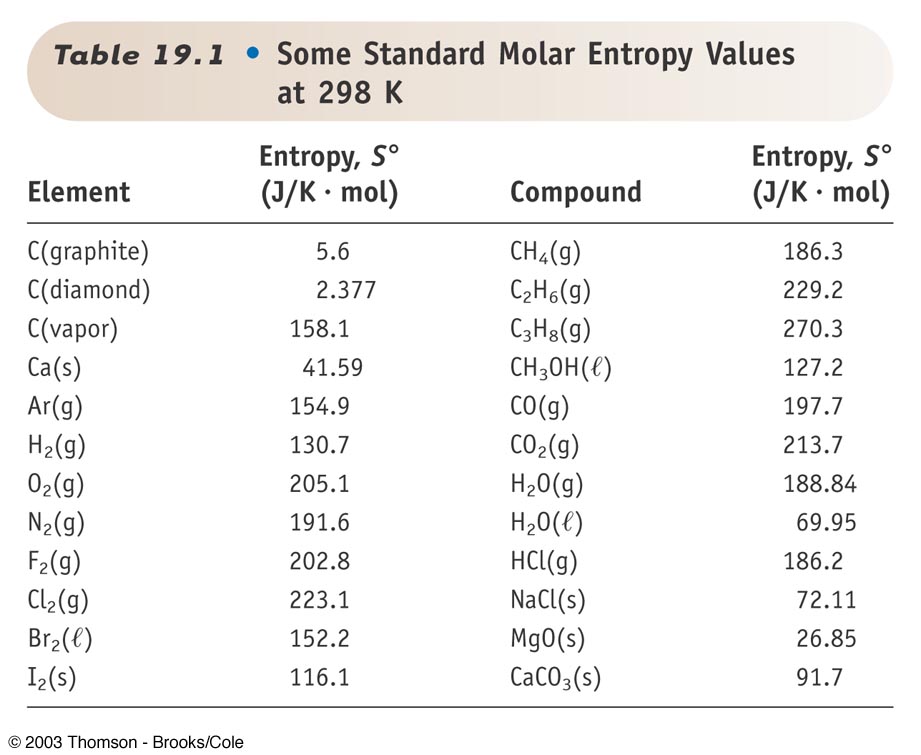
SOLVED: 9. (4 points) Which of the following has an absolute entropy equal to zero? H2O(s) at 273 K Hg(h) at 300 K 02(g) at 298 K all of the other choices

OneClass: Order the following substances from lowest (1) absolute entropy to highest (6). CO_2 (g) H_...

How to Calculate the Entropy Change for a Chemical or Physical Process Based on Absolute Entropies | Chemistry | Study.com
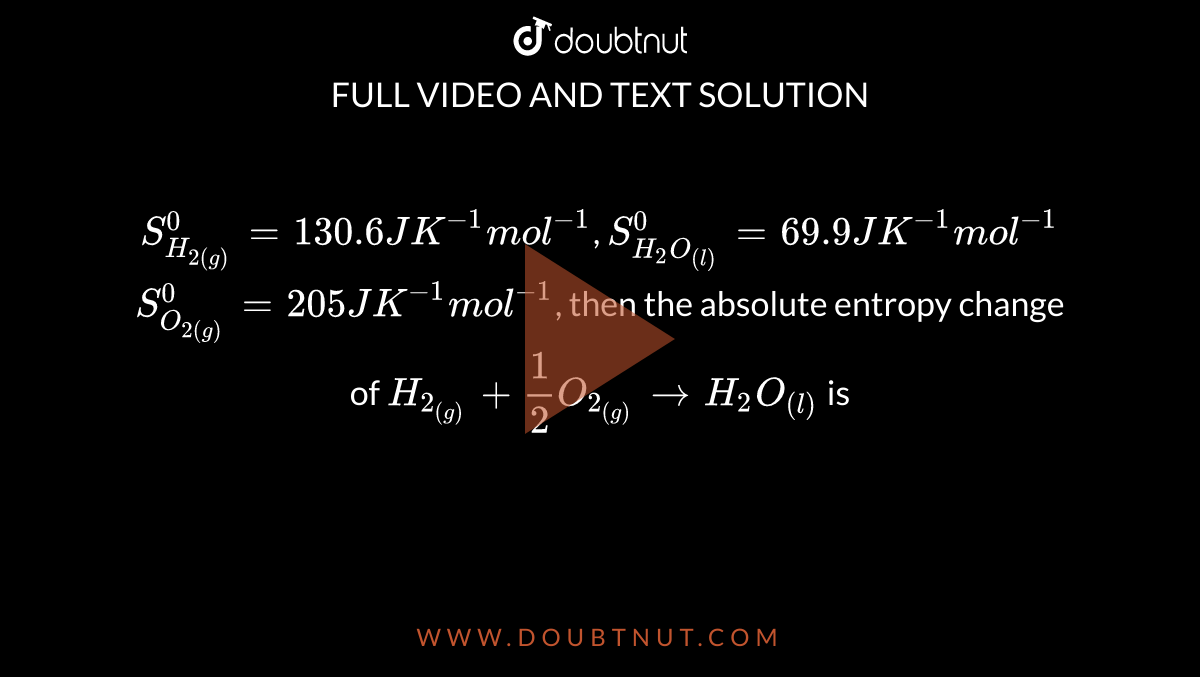
S(H(2(g)))^(0) = 130.6 J K^(-1) mol^(-1), S(H(2)O((l)))^(0)= 69.9 J K^(-1)mol^(-1) S(O(2(g)))^(0) = 205 J K^(-1)mol^(-1), then the absolute entropy change of H(2((g))) + (1)/(2)O(2((g))) rarr H(2)O((l)) is

Table V from Absolute entropy and free energy of fluids using the hypothetical scanning method. II. Transition probabilities from canonical Monte Carlo simulations of partial systems | Semantic Scholar

Why is negative the absolute entropy, enthalpy and internal energy in solids on the sublimation curve (steam properties)? | ResearchGate

Molar specific absolute entropy and molar specific Gibbs function of... | Download Scientific Diagram